Introduction
Ecological components of water are very significant for all existing organisms and the future of the earth. Every organism in the environment is linked to each other with a live connection. Thus, the deterioration that arises in a part of the system disturbs the whole structure over time. Water is the main source of life and cannot be replaced by other environmental components.
There are two main water bases on our planet. The first sources of water are surface sources called oceans, rivers, lakes and ponds. Surface water is a basic need for various classes of plants and animals that depend on the quantity and also depend on the quality of the water to live on the earth. The second source is groundwater, which is stored under the earth’s surface in aquifers. This groundwater feeds our ponds, rivers and oceans and provides drinking water for biosphere.
These two sources of water resources are serious to life on earth, both can become contaminated in dissimilar ways. Maintenance of surface water quality is important for the earth’s surface. It is caused by many factors, vegetation, atmospheric chemistry, geology, and anthropogenic activities. Mineral solubility in water causes extreme amounts of certain species of chemicals in natural waters. Carbonates are evaporating and dissolve rapidly and change the structure of water quicker, while other minerals silicates dissolve gradually and have fewer conspicuous effects on the structure of water. Most of the difficulties that people are facing in the current years are associated water quantity and water quality. Water should be preserved and protected from all kinds of contaminants. Water is an essential ingredient for the life. But today’s water resources are polluted by various toxic substances, industrial impurities and anthropogenic activities that result in some difficulties, such as bathing and irrigation activities; moreover, this leads to water shortages for humans and the environment.
Temple pond
- Temple ponds are the water reservoirs built as part of the temple complex. Most of the temple tanks having perennial water source help to keep the surroundings moist and cool. It recharges the underground water.
- Temple Ponds are traditional rain water storage structures built near the temples. In olden times, the water in the ponds was used for drinking purpose. In recent days it is used for bathing, washing and poojas.
- Temples serve as grounds for social and cultural interactions between peoples. The maintenance of healthy temple pond water needs proper monitoring of temple ponds. It will prevent the further deterioration of pond water.
Importance of temple pond water study
- Temples provide a peaceful environment with Vedic chants and ringing bells. Water is the source of life, absorbing positive energy. The energy of Vedic chants and rituals reside in the temple pond water, touching or bathing in those sacred water will give new energy.
- The good quality of water will maintain the good eco system in and around temple pond.
- Kanchipuram is a temple city, now a day’s temple ponds has reduced water level, increased pollution due to anthropogenic activities, seasonal variation. Hence, it is important to analyze the temple pond water.
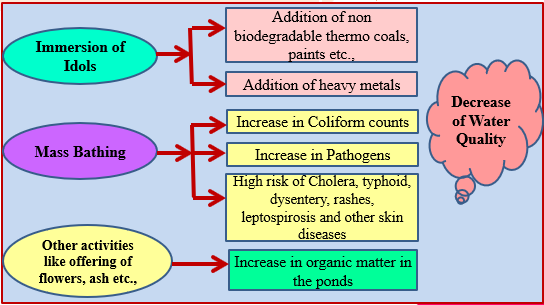
Sources of temple pond pollution
Temple pond pollution was primarily created due to anthropogenic activities, the main sources of pollution shown in Figure 1. Kailasanathar temple pond; the pollution created is due to poojas done in the pond, bathing and surrounding water discharged into the pond.
Study area
The study area was selected in Kanchipuram city. The study area temple pond has an elevation of 83.2 m (273 ft) above sea level. Kanchipuram is one of the prominent temple cities in South India. It is a conservative and tourist place, with a population of more than 1,70,000 people. This temple, whose presiding deity is Kailasanathar and whose mother is Kamakshi, is 1000 years old and constructed by pallavas. The place was called shiva linga medu in ancient times and corrupted to sevilimedu to the present day. The temple is located about 5.5 Kms from Kanchipuram. The pond present inside the temple is called Raahu Ketu theertham. It is on the north side of the temple. Two wells are present inside the pond. Poojas and thepam festival were conducted in the temple pond. This temple is called as Rahu Ketu sthalam.
Temple Pond Details
Latitude : 12050’32.20″N
Longitude : 79041’23.16″E
Size : 17m x 17m
Area : 289 m2
Activities : Temple usage, pooja activities, holy dip
Sampling of Water
The water sample was collected in the temple pond during the pre-monsoon (April), monsoon (September), and post monsoon (December) periods in the year 2018. The condition of pond water pre-monsoon, and post monsoon, as shown in Figure 2 and 3. A clean one litre polythene water bottle was taken, and it was immersed in pond water and rinsed two times. The sample water was collected up to the top level of the bottle, which was then tightly closed. The water sample was then transferred to the laboratory for testing of various water quality parameters. The standards and the measured water quality parameters are shown in Table 1 and Table 2.
Table 1. Water Quality Parameters and Surface Water Standards
| ISI – IS 2296 – 1982 Tolerance Limits for Surface Water in Various Classes (A,B,C,D,E) | ||||||||
| Sl.No | Water Quality Parameters (WQP) | Unit | A | B | C | D | E | |
| pH | pH | 6.5 – 8.5 | 6.5 – 8.5 | 6.5 – 8.5 | 6.5 – 8.5 | 6 – 8.5 | ||
| Electrical Conductivity | EC | µmho/cm | – | – | – | 1000 | 2250 | |
| Colour | Colour | Hazen | 10 | 300 | 300 | – | – | |
| Dissolved Oxygen | DO | mg/l | 6 | 5 | 4 | 4 | – | |
| Biological Oxygen Demand | BOD | mg/l | 2 | 3 | 3 | – | – | |
| Total Dissolved Solids | TDS | mg/l | 500 | – | 1500 | – | 2100 | |
| Chloride | Cl | mg/l | 250 | – | 600 | – | 600 | |
| Sulphate | SO4 | mg/l | 400 | – | 400 | – | 1000 | |
| Total Hardness | TH | mg/l | 300 | – | – | – | – | |
| Calcium | Ca | mg/l | 200 | – | – | – | – | |
| Magnesium | Mg | mg/l | 100 | – | – | – | – | |
| Fluoride | F | mg/l | 1.5 | 1.5 | 1.5 | – | – | |
| Ammonical Nitrogen | AN | mg/l | – | – | – | – | – | |
| Total Nitrogen | TN | mg/l | – | – | – | – | – | |
| Phosphate | P | mg/l | – | – | – | – | – | |
| Silica | S | mg/l | – | – | – | – | – | |
| Iron | Fe | mg/l | 0.3 | – | 50 | – | – | |
| Arsenic | As | mg/l | 0.05 | 0.2 | 0.2 | – | – | |
| Lead | Pb | mg/l | 0.1 | – | 0.1 | – | – | |
| Copper | Cu | mg/l | 1.5 | – | 1.5 | – | – | |
| Zinc | Zn | mg/l | 15 | – | 15 | – | – | |
| Total Coliform Count | TCC | MPN | 50 | 500 | 5000 | – | – | |
Class A – Drinking water source without conventional treatment but after disinfection.
Class B – Out door bathing.
Class C – Drinking water source with conventional treatment but after disinfection.
Class D – Fish culture and wild life propagation.
Class E – Irrigation, industrial cooling or controlled waste disposal.
Table 2. Measured Water Quality Parameters Values in Different in Seasons
| WQP | PRM Values | Class of Water in Pre Monsson | MON Values | Class of Water in Monsoon | POM Values | Class of Water in Post Monsoon | ||||||||||||
| A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | ||||
| pH | 7.47 | 7.6 | 9.31 | |||||||||||||||
| EC | 248 | 754 | 252 | |||||||||||||||
| Colour | Nil | Nil | Nil | |||||||||||||||
| DO | 7.3 | 6.3 | 6.3 | |||||||||||||||
| BOD | Nil | Nil | Nil | |||||||||||||||
| TDS | 161 | 428 | 164 | |||||||||||||||
| Cl | 23 | 80 | 41 | |||||||||||||||
| SO4 | 10 | 8.9 | 19 | |||||||||||||||
| TH | 166 | 160 | 90 | |||||||||||||||
| Ca | 22 | 32 | 13 | |||||||||||||||
| Mg | 27 | 19 | 14 | |||||||||||||||
| F | Nil | Nil | Nil | |||||||||||||||
| AN | 1.7 | Nil | 4.2 | |||||||||||||||
| TN | 7.5 | Nil | 12.9 | |||||||||||||||
| P | 7.2 | Nil | 8.3 | |||||||||||||||
| S | Nil | Nil | Nil | |||||||||||||||
| Fe | 0.72 | Nil | 0.04 | |||||||||||||||
| As | <0.01 | Nil | <0.01 | |||||||||||||||
| Pb | <0.01 | Nil | <0.01 | |||||||||||||||
| Cu | <0.01 | Nil | <0.01 | |||||||||||||||
| Zn | <0.01 | Nil | <0.01 | |||||||||||||||
| TCC | 500 | Nil | 1600 | |||||||||||||||
| Measured values with in Standard limit | |
| Measured values greater than in Standard limit | |
| Measured values with in Safe limit |
Result and discussion
- The pH of a water indicates the concentration of hydrogen ion. pH is a sign of the survival of biological life. Measured values indicate the temple pond water is within safe limit for the pre-monsoon and monsoon period, in the post monsoon period, the water has a greater pH value due to the discharge of all surrounding water into the pond.
- Electrical conductivity values vary depending on the various ions present in the water body, their comparative concentrations, and the ionic strength. EC is a helpful tool to assess the purity of water. Class A, B, and C no limit given for electrical conductivity Given that the pond water is safe in three seasons, the class D, E limit is met.
- The dissolved oxygen plays a most significant role in the survival of aquatic life. The existence of DO is essential to preserving the higher forms of natural life and keeping an appropriate balance of the various pollutions, thus keeping the water bodies healthy. The measured values were greater than the standard limit in three seasons. The DO is acceptable for 6 to 8.5 mg/l for pond water. Hence, the pond water is safe in all three seasons.
- The total dissolved solids are the suspended and dissolved matter present in water. In three seasons, the measured TDS values were within given standard limit according to classes A, C, and E.
- Chloride is a natural element; hydrogen chloride is in its dissolved form. It is present in nature as a salt. In three seasons, the measured chloride values were within given standard limit according to classes A, C, and E.
- Sulphate ions are naturally present in water. Sulphate is mixed into pond water by infiltrating waters and surface runoff. In three seasons, the measured sulphate values within the given standard limit according to classes A, C, and E.
- The hardness of water is a measure of its capability to form precipitates with soap and scales with certain anions present in the water. The limit given for class A only includes measured values less than 600 mg/l in three seasons. The pond water is safe in all classes according to hardness.
- Calcium serves as one of the important micro nutrients in organisms. The limit given for class A only includes measured values less than 200 mg/l in three seasons. The pond water is safe in all classes according to calcium.
- Magnesium is commonly found to be associated with calcium in all variety of waters, but its deliberation remains generally lesser than that of calcium. For chlorophyll growth, magnesium is needed. The limit given for class A only includes measured values less than 100 mg/l in three seasons. The pond water is safe in all classes, according to magnesium.
- Ammonical Nitrogen is extremely soluble in water, reacting with water to produce ammonium hydroxide, it is part of the nitrogen cycle, which is influenced by biological activity.
- Total Nitrogen is an essential nutrient for plants and animals. An excess amount of nitrogen in a waterway may lead to low levels of dissolved oxygen and negatively alter various plant life and organisms.
- Phosphorus is an essential nutrient for plants and animals. Excessive phosphorus in surface water can cause explosive growth of aquatic plants and algae. This can lead to a variety of water-quality problems, including low dissolved oxygen concentrations, which can cause fish kills and harm other aquatic life.
- Ammonical nitrogen, total nitrogen and, phosphate and silica limits are not given, hence the pond water safe in three seasons according to all classes.
- Iron is the nutrient element for humans. It is an important mineral for the production of haemoglobin. The heavy metal iron limit for class A is 0.3 mg/l, in pre-monsoon the measured value greater than the limit, other classes with in the limit. In the monsoon and post monsoon period; the pond water is safe from content according to class B, C, D, E.
- The remaining heavy metals arsenic, lead, copper and, zinc is within the standard limit. Hence, the pond water is safe from these heavy metals.
- A microbiological test is used to detect the level of pollutions caused by living things. This produces pathogenic diseases on fish. The class C standard maximum value is 5000 MPN. The measured values indicate, according to class A, that the pond water has greater (50 MPN) value of coliform count in pre-monsoon and post monsoon. In post monsoon, the value was greater (500 MPN) than class B standards. The result indicates the pond water not directly useful for drinking purpose.
Conclusion
The Kailasanathur temple pond water quality was studied in the three seasons. The result showed that in the pre – monsoon period, the iron value was 0.72 mg/l and the coliform count was 500 MPN greater than class A surface water standards, it was not directly used for drinking purposes. It is safe in class B, C, D, and E. The pond water is safe for bathing, fish culture, irrigation, gardening, and drinking after purification with conventional treatment followed by disinfection. According to the monsoon period, all the measured values were within limits according to all classes, hence, it is directly useful for drinking and other purpose. The result compared to post monsoon period is that the pH value is greater (9.31) than the standard limit according to all the classes. The coliform count value was greater 1600 MPN in compared to classes A and B. The pond water, with proper treatment, can be useful for all purposes. Hence, the study indicates that rain water will purify all impurities in the pond. Protection of pond and avoiding mixing of surrounding water into the pond will provide safe water in the temple pond.